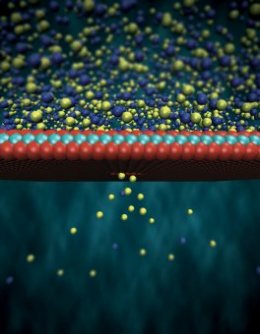
 A molybdenum 3-atoms-thick discerning membrane layer. Credit: © Steven Duensing / nationwide Center for Supercomputing Applications, University of Illinois, Urbana-Champaign
A molybdenum 3-atoms-thick discerning membrane layer. Credit: © Steven Duensing / nationwide Center for Supercomputing Applications, University of Illinois, Urbana-Champaign
Supporters of clean energy will quickly have a new supply to enhance their existing assortment of solar power, wind, and hydropower: osmotic power. Or maybe more particularly, energy produced by an all natural occurrence happening whenever fresh water makes contact with seawater through a membrane.
Scientists at EPFL's Laboratory of Nanoscale Biology are suffering from an osmotic energy generation system that provides never-before-seen yields. Their development lies in a three atoms thick membrane regularly separate the 2 liquids. The results of their analysis have now been published in Nature.
If the system is employed with seawater and fresh-water, sodium ions into the seawater go through the membrane layer into the fresh-water until both liquids have a similar sodium concentration. And because an ion is simply an atom with a power charge, the movement of this sodium ions are harnessed to build electrical energy.
A 3 atoms thick, selective membrane that does the job
EPFL's system is made of two liquid-filled compartments separated by a slim membrane layer made of molybdenum disulfide. The membrane has a tiny gap, or nanopore, by which seawater ions pass to the fresh water through to the two fluids' salt levels are equal. Whilst the ions go through the nanopore, their electrons tend to be utilized in an electrode - that is understanding regularly generate an electric present.
As a result of its properties the membrane layer permits positively-charged ions to pass through, while pressing away a lot of the negatively-charged ones. That produces voltage involving the two liquids as you builds an optimistic cost while the other a poor cost. This current is what triggers current produced by the transfer of ions to move.
"we'd to first fabricate then explore the perfect size of the nanopore. If it's too large, unfavorable ions can move across as well as the resulting voltage would be also low. If it is too little, not enough ions can go through and also the existing could be too weak, " stated Jiandong Feng, lead author of the research.
The video clip will load immediately
Exactly what sets EPFL's system apart is its membrane. During these types of methods, the present increases with a thinner membrane. And EPFL's membrane is simply a few atoms dense. The materials it's made of - molybdenum disulfide - is fantastic for producing an osmotic existing. "This is basically the very first time a two-dimensional product has been utilized for this particular application, " said Aleksandra Radenovic, mind associated with the laboratory of Nanoscale Biology
Powering 50'000 energy-saving bulbs with 1m2 membrane
The possibility regarding the brand-new system is huge. Based on their computations, a 1m² membrane with 30per cent of their surface included in nanopores should be able to produce 1MW of electricity - or adequate to power 50, 000 standard energy-saving bulbs. And because molybdenum disulfide (MoS2) is easily found in nature or is grown by substance vapor deposition, the system could feasibly be ramped up for large-scale energy generation. The major challenge in scaling-up this technique is finding-out steps to make relatively uniform pores.
Up to now, scientists been employed by on a membrane with an individual nanopore, to realize just what had been taking place. '' From a manufacturing point of view, single nanopore system is ideal to help our fundamental comprehension of membrane-based processes and offer useful information for industry-level commercialization'', said Jiandong Feng.
The researchers could operate a nanotransistor through the current generated by one nanopore and thus demonstrated a self-powered nanosystem. Low-power single-layer MoS2 transistors were fabricated in collaboration with Andreas Kis' group at at EPFL, while molecular dynamics simulations had been performed by collaborators at University of Illinois at Urbana-Champaign
Harnessing the potential of estuaries
EPFL's research is part of an increasing trend. For past years, boffins worldwide were developing methods that control osmotic capacity to develop electrical energy. Pilot jobs have actually sprung up in locations eg Norway, the Netherlands, Japan, and united states of america to come up with energy at estuaries, in which streams flow to the ocean. For now, the membranes used in many systems are organic and fragile, and provide reasonable yields. Some methods utilize the movement of water, as opposed to ions, to power turbines that consequently produce electrical energy.
When the systems be a little more powerful, osmotic energy could play a significant role in generation of green power. While solar power panels require adequate sunlight and wind generators sufficient wind, osmotic energy are produced virtually any time or evening - supplied there is an estuary nearby.